Addressing the world’s most pressing cancer treatment challenges
Software is the driving force in today’s IGT-image guided therapy market adding a never seen before precision and confidence to the clinical practice.
Today, when tumors are treated with minimally invasive procedures – they fail too often. More then half of treatments are unable to completely eliminate cancerous tissue.
BioTrace by Techsomed provide a 360 degree intelligent view compressing thousands of imaging frames into one actionable interface in one system in real time.
When visibility is limited, BioTrace™ platform enhances the ablation workflow, equipping physicians with cutting edge visualization, AI-powered tools and insight they need to treat with clarity and confidence from planning al the way to treatment confirmation.
World’s First FDA-Cleared Software for tissue response prediction.
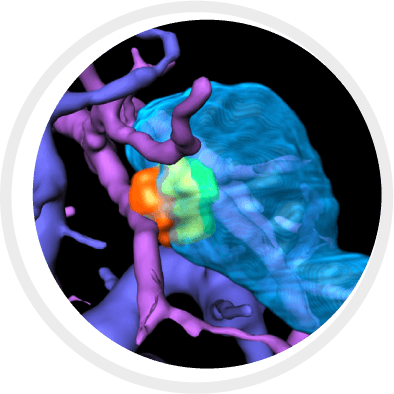
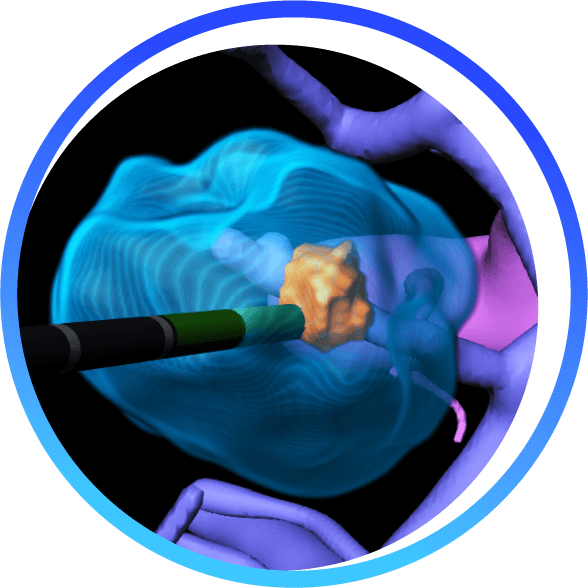
BioTrace is compressing thousands of imaging frames into one actionable interface, overlaying risk-aware 3D models to deliver smarter needle paths, real-time margin visualization, In-Procedure tumor coverage assessment and post-ablation tissue response predictions.
The immediate clinical impact
The Only Full-Cycle Ablation Management, Offering AI-driven Insights for Precise Tumor Margin Control.
The BioTraceIO360 solution comprises the BioTraceIO Vision and BioTraceIO Precision software products, both have been FDA-cleared for marketing in the U.S.
Why achieving clear margins improves clinical outcomes?
Supporting clinical references:
Remove uncertainty for optimal results with real-time, informed decision-making
Enhanced Coverage & Complication Detection
Multi-Modal
Registration
The First FDA-Cleared Tissue Response Prediction (TRP)
Real-Time, interactive tumor coverage Quantitative Feedback
Vendor-Agnostic
Integration
Tackle challenges in under-utilized verticals in minimally invasive therapies through AI-powered smart software tools with the goal of making them accessible for everyone, everywhere.

Yossi Abu




Nitzan Even

Yogev Zohar

Dubi Finklshtein

Tobias Preusser

Limor Shimoni
Investors
Partners
Clinical



Hadassah and TechsoMed Partner to Enhance Cancer Treatment with Advanced AI-Driven Image-Guided Therapy Solutions Powered by Big Data.
